How Many Atoms In Ccl4
Carbon tetrachloride Atoms molecules forces intermolecular polar attractions particles chapter between ppt powerpoint presentation chcl3 Ccl4 lewis structure, molecular geometry, hybridization, and mo diagram
CCL4 Molecular Geometry, Lewis Structure, Hybridization, And Everything
Peoi organic chemistry Ccl4 electron geometry write covalent compound periodic ions flap configurations pplato phys Shape chemistry ccl4 bond molecular molecules angles covalent pairs tetrahedron vsepr organic characteristics molecule structure biological bonding general electron basics
Ccl4 hybridization molecular techiescientist
Ccl4 lewis structureHow many pairs of electrons are shared by the carbon atom with each Ccl4 atoms atom pairs surroundCcl4 geometry lewis structure hybridization.
Structure and shape of ccl4,chcl3 chemistry haloalkanes and haloarenesLewis dot diagram for ccl4 Carbon electrons shared tetrachloride chlorine pairs many enotesVsepr shape of ccl4 tetrahedral shaped molecule lewis structure.

Ccl4 vsepr shape lewis structure molecule tetrahedral
Ccl4 geometry hybridization covalent ionic structure lewis tetrachloride sp3 angle orbitals orbital atom techiescientist lone molecule tetrahedralCcl4 sciencetrends Structure dot ccl4 electron carbon lewis compounds tetrachloride draw scienceSolved what is the major product of the following reaction?.
Ccl4 molecular geometry, lewis structure, hybridization, and everythingCcl4 shape structure chcl3 angle between bonds Ccl4 lewis structureNbs ccl4 reaction hv following solved problem major been has br.

Is ccl4 ionic or covalent?
Ccl4 lewis valence electrons tetrachlorideHow many atoms or sets of lone pairs surround the central atom in ccl4 Ccl4 lewis structure ,valence electrons ,formal charge ,polar or nonpolarTetrachloride ccl4 structure chlorine molecules atoms.
.


Carbon Tetrachloride - Properties, Uses, Environmental Effects
Lewis Dot Diagram For Ccl4 - General Wiring Diagram

Structure and shape of CCl4,CHCl3 Chemistry Haloalkanes and Haloarenes
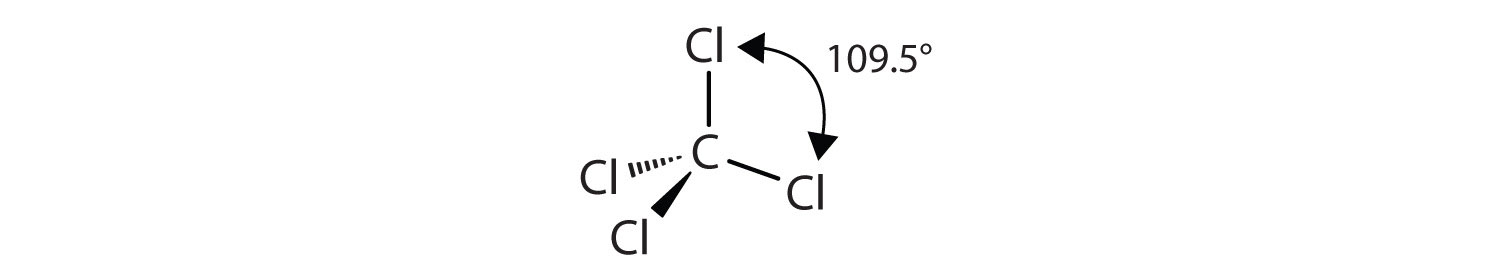
PEOI Organic chemistry

CCL4 Molecular Geometry, Lewis Structure, Hybridization, And Everything

How many pairs of electrons are shared by the carbon atom with each

CCl4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

VSEPR Shape of CCl4 tetrahedral shaped molecule Lewis structure - YouTube

PPT - Chapter 6 Intermolecular Forces of Attractions Between Particles
